-
Since Bekenstein and Hawking [1, 2] demonstrated that black holes can be observed as thermodynamic objects with temperature, it is widely accepted that black hole thermodynamics has been an exciting and challenging field in theoretical physics. As fascinating phenomena in black hole thermodynamics, phase transitions and critical behaviors have attracted the attention of several researchers. Based on the analogy between black holes and thermodynamic objects, Hawking and Page proved the existence of a phase transition between the Schwarzschild-AdS black hole and thermal AdS space [3]. After their pioneering research, several studies were conducted in this direction, and rich phase structures were discovered [4−8]. The later-established AdS/CFT correspondence has further stimulated attention toward asymptotically anti-de Sitter (AdS) black holes [9, 10]. In different AdS black hole backgrounds, phase structures and critical phenomena have been studied and promoted [11−19].
Note that the energy definition of Kerr-AdS black holes is not unique. This is because the standard Komar energy expression diverges at infinity. Henneaux and Teitelboim [20] first derived the energy
m/Ξ2 , which satisfies the first law of black hole thermodynamics. The authors [21] proposed the energym/Ξ3/2 by applying the Hamiltonian approach. Using the Iyer-Wald formalism, Gao et al. [22] clarified the origins of the two different energy definitions of Kerr-AdS black holes. They found the energies associated with different observers at infinity, and there is a relative rotation between the two types of observers. The associated thermodynamics for the different energies are also obtained in [22]. For non-rotating and rotating observers, the energies arem/Ξ2 andm/Ξ3/2 , respectively. The energym/Ξ2 corresponds to the standard thermodynamics of black hole, and its related critical phenomena have been studied, including phase transition and critical exponents [16]. The other energym/Ξ3/2 is related to the modified first law of black hole thermodynamics [22]. Considering that the phase transition is associated with the degrees of freedom within the system, and there is only a relative rotation between two observers, it is reasonably conjectured that there should be similar phase structures in modified thermodynamics. In the current study, we investigate whether the phase transition structure exists under the modified first law of thermodynamics. Furthermore, the observer dependence of the phase structure is discussed.This paper is organized as follows. Section II briefly reviews the basic knowledge of the Kerr-AdS black hole. Section III discusses the modified first law and thermodynamic quantities. The main results are presented in Sections IV and V; the phase structure of the modified first law is shown. Discussions are given in the last section.
-
The Kerr-AdS black hole solution of the Einstein equations in the Boyer-Lindquist coordinates is expressed as
ds2=−Δρ2(dt−asin2θΞdϕ)2+ρ2Δdr2+ρ2Σdθ2+Ξsin2θρ2(adt−r2+a2Ξdϕ)2,
(1) with
ρ2=r2+a2cos2θ,Ξ=1−a2l2,Σ=1−a2l2cos2θ,Δ=(r2+a2)(1+r2l2)−2mr.
(2) Here,
Λ=−3l−2 is the cosmological constant,m,a are the mass parameter and angular momentum parameter, respectively. The associated thermodynamic quantities are [16]T=3r4++(a2+l2)r2+−l2a24πl2r+(r2++a2),S=π(r2++a2)Ξ,ΩH=aΞr2++a2,
(3) where
r+ represents the horizon radius satisfyingΔ(r+)=0 ; T, S, andΩH are defined as the Hawking temperature, Bekenstein-Hawking entropy, and angular velocity, respectively. The energy M and angular momentum J areM=mΞ2 andJ=amΞ2 , respectively.In 2012, S. Gunasekaran et al. [23] introduced the extended phase space for AdS black hole, where the cosmological constant Λ can be interpreted as a pressure term via the following relation:
P=−Λ8π.
(4) The first law of black hole thermodynamics and the Smarr formula are
δM=TδS+ΩHδJ+VδP,
(5) M2=TS+ΩHJ−VP,
(6) where the thermodynamic volume V is
4πl2r+(a2+r2+)3(−a2+l2) . Following Gunasekaran et al.'s perspective, several studies have focused on exploring the thermodynamic properties of AdS black holes on the extended phase space [24−27]. -
There is ambiguity on the energy notion of Kerr-AdS black holes [20, 21], which has been a long standing issue. In the recent article [22], the authors proposed a natural criteria to justify the notion of energy. In particular, they examined whether the associated first law of the black hole thermodynamics exists. Within the Iyer-Wald formalism, two versions of the first law and the Smarr formula were established for different energies. The difference originated from the choice of the Killing vectors. The standard energy notion
m/Ξ2 is associated with the Killing vector∂∂T=∂∂t+13aΛ∂∂ϕ . The relevant thermodynamic quantities, first law, and standard results are presented in Section II. The other energy notionˆM=m/Ξ3/2 is related to the Killing vector1√Ξ∂∂t . By employing the notation from [22], the corresponding thermodynamic quantities can be expressed asˆΩH=ΩH√Ξ,ˆT=T√Ξ,ˆV=V√Ξ.
(7) The modified first law of black hole thermodynamics is presented as
δˆM=ˆTδS+ˆΩHδJ+ˆVδP.
(8) Based on the concrete expressions of the Killing vectors, there exists a relative rotation between two types of observers. Contrary to a naive observation,
∂∂T coincides with the generator of the conformal boundary, corresponding to non-rotating observers, whereas rotating observers are related to∂∂t . -
Using Eq. (7), the functions
ˆΩH , J, andˆT can be expressed asJ=3a(a2+r2+)(3+8Pπr2+)2(3−8a2Pπ)2r+,
(9) ˆΩH=2a√P(−a2+38Pπ)√2π3a2+r2+,
(10) ˆT=√19−24a2Pπ[a2(−3+8Pπr2+)+3(r2++8Pπr4+)]4πr+(a2+r2+).
(11) The above equations can be viewed as equations of state of the Kerr-AdS black hole.
In this section, we focus on the phase structure of modified black hole thermodynamics on the
(ˆΩH,J) plane. A previous study [16] had considered similar problems for standard black hole thermodynamics on the(J,ΩH) section, without considering the extended phase space.According to the equations of state (9)−(11), the isotherms for various temperatures on the
ˆΩH−J plane are presented in Fig. 1. Qualitatively, Fig. 1 is similar to the liquid/gas PVT diagram [28]. Under the following correspondence,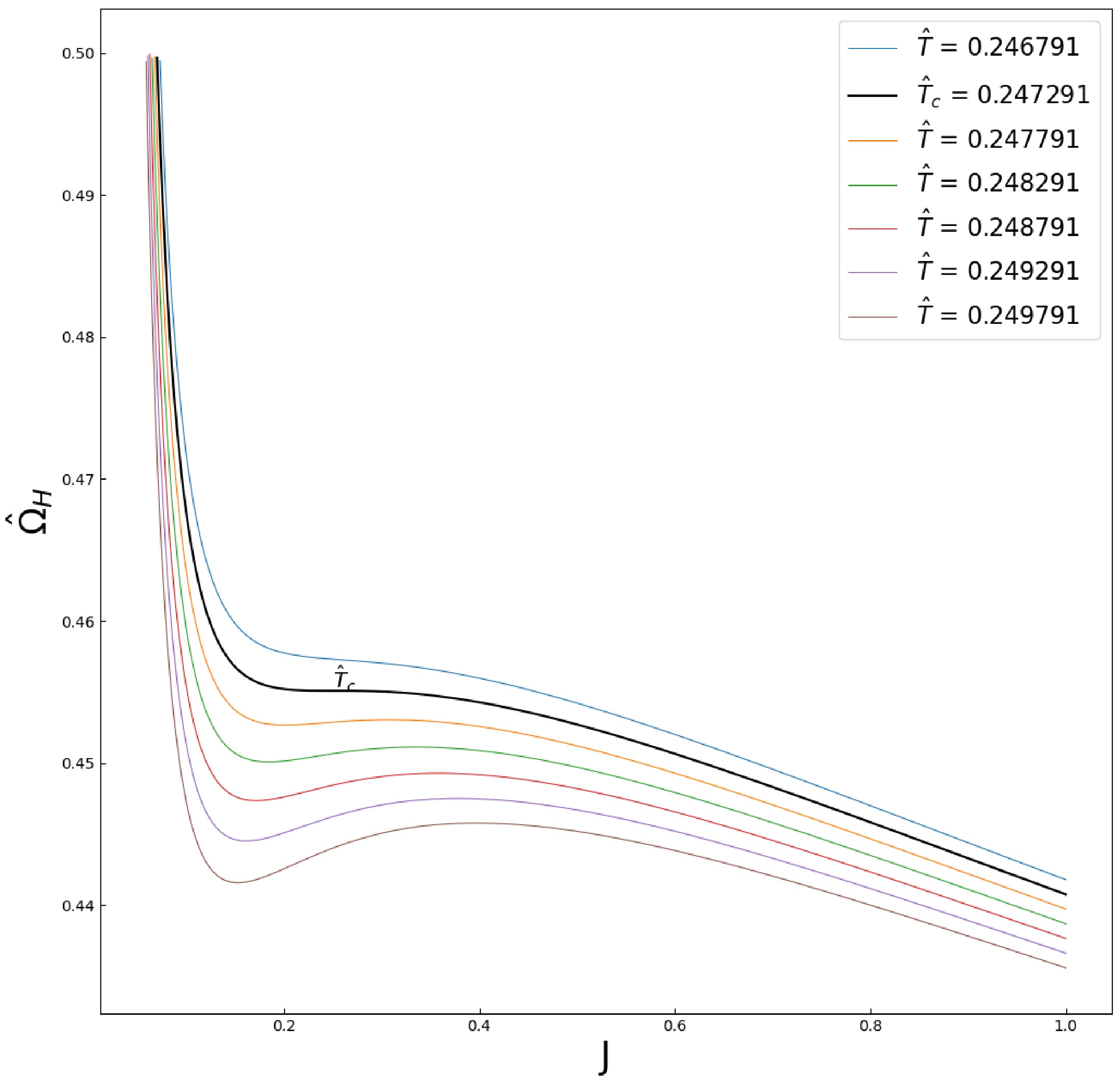
Figure 1. (color online) Isotherms of the Kerr-AdS black hole with
l=1 in theˆΩH−J plane. Qualitatively, the isotherms near the critical temperatureˆTc behave similar to a van der Waals system. The black solid line is the critical isotherm.ˆΩH→P,J→V,
(12) there is a van der Waals-like phase structure. By contrast, the correspondence in the previous article [16] was
J→P,ΩH→V .As the temperature decreases until
ˆTc , an inflection point is formed. Therefore, the temperatureˆTc and angular velocityˆΩH at the critical point satisfy [28](∂ˆΩH∂J)ˆTc=0,(∂2ˆΩH∂J2)ˆTc=0.
(13) Combining with the equations of state (9)−(11), the critical point is
Jc=3(4+√2)(1+2√2)3/2√−5+4√2(−6+5√2)448(−3+√2)2Pπ,
(14) ˆΩHc=2√23(−23+17√2)πP(−2+3√2),
(15) ˆTc=7(−2+3√2)√2P9π−3√2π(1+2√2)3/2(−6+5√2).
(16) -
In this subsection, we first discuss the coexistence condition between two states, from which the law of equal area is obtained. For two stable states,
ˆa andˆb , it is well-known that the coexistence condition is represented by the free energy being equal [28], i.e.,G(ˆa)=G(ˆb) . Compared with the PTV system, the free energy for the black hole system should bedG=−SdˆT+JdˆΩH.
(17) Fixing the temperature, one can obtain the free energy by integrating the above equation along the isothermal curve. The difference in free energy between two states should be
∫ˆbˆaJdˆΩH (this integral is along the isothermal curve that connects the two states). From Fig. 2(a), becauseˆa andˆb are coexistent states,
Figure 2. (color online) (a) The brown solid line represents an isotherm with temperature
ˆT=0.248291 andl=1 ;ˆa andˆb are coexistence states. Because of the equal free energy requirement of coexistence states, i.e.,G(ˆa)=G(ˆb) , area(A) = area(B). (b) The red dashed line is the coexistence curve of phase transition aboveˆTc . The black solid line represents the critical isotherm withl=1 .G(ˆa)−G(ˆb)=∫ˆΩHˆbˆΩHˆaJdˆΩH=0,
(18) implying that the areas of region A and region B in Fig. 2(a) are equal, corresponding to the Maxwell equal area law.
To investigate the critical behavior near the critical point, we should define the order parameter. Analogous to the van der Waals system,
η=Jb−Ja2 is defined as the order parameter. The coexistence curve is the red dashed line in Fig. 2(b). -
For a van der Waals system, near the critical point, one can obtain the critical exponents [28]. For the correspondence (12), the analogous critical exponents can be obtained as follows.
∙ Degree of critical isotherm:ˆΩH−ˆΩHc=Aδ|J−Jc|δsign(J−Jc),ˆT=ˆTc.
(19) ∙ Degree of coexistence curve:η=−Aβ(ˆT−ˆTc)β,ˆT>ˆTc.
(20) ∙ Degree of heat capacity(J=Jc ):CJ={Aα′{−(ˆT−ˆTc)}−α′,ˆT<ˆTcAα{+(ˆT−ˆTc)}−α,ˆT>ˆTc.
(21) ∙ Degree of isothermal compressibility:κT=−1J(∂J∂ˆΩH)ˆT={Aγ′{−(ˆT−ˆTc)}−γ′,ˆT<ˆTcAγ{+(ˆT−ˆTc)}−γ,ˆT>ˆTc.
(22) In the following subseciton, we calculate explicitly the critical exponents for modified black hole thermodynamics.
-
At the critical point, the first and second derivatives of
ˆΩH with respect to J satisfy(∂ˆΩH∂J)ˆTc=0,(∂2ˆΩH∂J2)ˆTc=0.
(23) The third derivative can be calculated as
(∂3ˆΩH∂J3)ˆTc=−16384(πP)7/29√87√2+123≠0,
(24) hence,
δ=3 according to the definition of δ in Eq. (19). -
In Fig. 2(b), we plot the curve of the coexisting states using Maxwell's equal-area law. Along this curve, all thermodynamical quantities depend only on temperature. To find the value of the degree of the coexistence curve, β, we should obtain the relations between η and
ˆT near the critical point. Therefore, we expandˆΩH in terms of J andˆT to the third order asˆΩH−ˆΩHc≈(∂ˆTˆΩH)J|c(ˆT−ˆTc)+12(∂2ˆTˆΩH)J|c(ˆT−ˆTc)2+(∂ˆT(∂JˆΩH)ˆT)J|c(ˆT−ˆTc)(J−Jc)+16(∂3ˆTˆΩH)J|c(ˆT−ˆTc)3+16(∂3JˆΩH)ˆT|c(J−Jc)3+12(∂2ˆT(∂JˆΩH)ˆT)J|c(ˆT−ˆTc)2(J−Jc)+12(∂ˆT(∂2JˆΩH)ˆT)J|c(ˆT−ˆTc)(J−Jc)2.
(25) For simplicity, let us introduce
ω=ˆΩH−ˆΩHc,¯t=ˆT−ˆTc,j=J−Jc,
(26) then, Eq. (25) becomes
ω=c10¯t+c20¯t2+c11¯tj+c30¯t3+c03j3+c21¯t2j+c12¯tj2.
(27) According to the equations of state (9)−(11), one can calculate all coefficients in Eq. (27) as
c10=−√2π,c20=√6(29√2−41)π3/2√1P,c11=643(√2−1)π2P, c30=9(11√2−15)π2P,c03=−16384(πP)7/29√87√2+123,c21=−544√2P5331√2+7539π5/2,c12=20483(4−3√2)π3P2.
(28) The equilibrium condition
ˆΩa=ˆΩb impliesω(ja,t)=ω(jb,t) , which in turn yieldsωb−ωa=(jb−ja)[c11¯t+c21¯t2+c12¯t(ja+jb)+c03(j2a+jajb+j2b)]=0.
(29) By employing Maxwell's equal area law, the area of the shaded region in Fig. 3 is equal to the area of the rectangle
cdˆbˆa in Fig. 3. Therefore, we obtain
Figure 3. (color online) According to the equal area law, i.e., area(A) = area(B), the area of the shaded region should be equal to the area of the rectangle
cdˆbˆa .∫JbJaˆΩdJ=(Jb−Ja)⋅12(Ωb+Ωa),
(30) The left side of Eq. (30) represents the area of the shaded region in Fig. 3 , and the right side represents the area of the rectangle cdba in Fig. 3. The factor
12(Ωb+Ωa) originates from the equilibrium conditionˆΩa=ˆΩb .) By using Eq. (26), Eq. (30) becomes∫JbJaˆΩdJ=[∫JbJa(ˆΩ−ˆΩc)d(J−Jc)]+ˆΩc[(Jb−Jc)−(Ja−Jc)]=[∫jbja(ˆΩ−ˆΩc)dj]+ˆΩc(jb−ja)=[∫jbjaωdj]+ˆΩc(jb−ja)=(Jb−Ja)12(ˆΩb+ˆΩa)=(jb−ja)12([ˆΩb−ˆΩc]+[ˆΩa−ˆΩc])+(jb−ja)ˆΩc=(jb−ja)12(ωb+ωa)+(jb−ja)ˆΩc.
(31) The above result implies that
∫jbjaωdj=(jb−ja)12(ωb+ωa).
(32) Substituting Eq. (27) into Eq. (32), one can obtain
∫jbja(c10¯t+c20¯t2+c11¯tj+c30¯t3+c03j3+c21¯t2j+c12¯tj2)dj=c10¯t(jb−ja)+c20¯t2(jb−ja)+12c11¯t(j2b−j2a)+c30¯t3(jb−ja)+14c03(jb+ja)(j2b+j2a)+12c21¯t2(jb+ja)+13c12¯t(j2b+jajb+j2a)]=(jb−ja)12(ωa+ωb),
(33) dividing both sides of Eq. (33) by
(jb−ja) yieldsc10¯t+c20¯t2+12c11¯t(jb+ja)+c30¯t3+14c03(jb+ja)(j2b+j2a)+12c21¯t2(jb+ja)+13c12¯t(j2b+jajb+j2a)=c10¯t+c20¯t2+12c11¯t(jb+ja)+c30¯t3+12c03(j3b+j3a)+12c21¯t2(jb+ja)+12c12¯t(j2b+j2a).
(34) Eliminating similar terms on both sides of Eq. (34) yields
14c03(jb+ja)(j2b+j2a)+13c12¯t(j2b+jajb+j2a)=12c03(j3b+j3a)+12c12¯t(j2b+j2a).
(35) Then, we can obtain
14c03(j3b+j2bja+jbj2a+j3a)+13c12¯t(j2b+jajb+j2a)=12c03(j3b+j3a)+12c12¯t(j2b+j2a),
(36) which implies
0=14c03(j3a−jaj2b−j2ajb+j3b)+16c12¯t(j2b−2jajb+j2a)=14c03(jb+ja)(jb−ja)2+16c12¯t(jb−ja)2.
(37) By simplifying the above equation, we can obtain
(jb−ja)2[14c03(ja+jb)+16c12¯t]=0.
(38) Denoting
j−≡jb−ja=Jb−Ja,j+≡jb+ja and considering Eq. (38),j+ can be solved asj+=−2c12¯t3c03,
(39) Substituting Eq. (39) into Eq. (29) yields
j−=√−4c11¯t+(4c2123c03−4c21)¯t2c03.
(40) Near the critical point, the temperature dependence of the order parameter is
Jb−Ja2≈Aβ(ˆT−ˆTc)1/2.
(41) Thus,
β=12 . -
According to definition (21), the critical exponent of heat capacity can be obtained as follows. Recall that the energy
ˆM is expressed byˆM=(a2+r2+)(r2+l2+1)2r+(1−a2l2)3/2,
(42) then, the heat capacity
CJ can be calculated asCJ=(∂ˆM∂ˆT)J|c=38P≠0.
(43) Since the heat capacity neither diverges nor vanishes, α and
α′ are both zero, i.e.,α=α′=0 . -
The isothermal comprssibility
κT is defined asκT=−1J(∂J∂ˆΩH)ˆT,
(44) which diverges at the critical point.
˜ω,˜t , and˜j are introduced as˜ω=ˆΩH−ˆΩHcˆΩHc,˜t=ˆT−ˆTcˆTc,˜j=J−JcJc,
(45) which yields
ˆΩH=ˆΩHc˜ω+ˆΩHc,ˆT=ˆTc˜t+ˆTc,J=Jc˜j+Jc,
(46) and
ω=ˆΩHc˜ω,¯t=ˆTc˜t,j=Jc˜j.
(47) Eq. (27) can be rewritten as
˜ω=~c10˜t+~c20˜t2+~c11˜t˜j+~c30˜t3+~c03˜j3+~c21˜t2˜j+~c12˜t˜j2.
(48) Using Eq. (44), we obtain
−1JκT=1(∂J∂ˆΩH)ˆT=(∂ˆΩH∂J)ˆT.
(49) Combining Eqs. (41), (46), (47), and (48) yields
−1JκT=(∂ˆΩH∂J)ˆT=(∂ˆΩH∂˜j∂˜j∂J)ˆT=ˆΩHcJc(∂˜ω∂˜j)˜t∝(∂ω∂j)¯t,
(50) namely
κT−1∝(∂ω∂j)¯t=c11¯t+3c03j2+o(¯t)=C1¯t+o(¯t),
(51) where
C1 is a real constant; Eq. (41) is used in the last step. Based on the definition of γ andγ′ in Eq. (22), we obtainγ=γ′=1 . -
According to the differential expression
dG=−SdˆT+JdˆΩH , at constant angular velocityˆΩH , we can employ Eqs. (3), (10), and (11) to plot theG−ˆT diagram.From Fig. 4, the swallowtail structure is analogous to the van der Waals system. This further confirms the similarity between the modified thermodynamics and the PVT system.

Figure 4. (color online) Qualitative behavior of free energy as a function of temperature for various angular velocities with
l=1 . The angular velocity decreases from bottom to top. The green solid line corresponds toˆΩH>ˆΩHc , the yellow solid line representsˆΩH=ˆΩHc , and the remaining red solid lines correspond toˆΩH<ˆΩHc . ForˆΩH<ˆΩHc , the existence of the swallowtail structure implies that a first-order phase transition occurs in the system. -
It is well-known that, in the vicinity of the critical point, the free energy can be expressed as a homogenous function, with corresponding homogenous indices p and q [28]. Based on the previous discussions, we observe that the critical exponents satisfy the following expected relations:
α+2β+γ=2,α+β(γ+1)=2,
(52) γ(δ+1)=(2−α)(δ−1),γ=β(δ−1).
(53) The free energy has the following scaling symmetry
gs(Λpϵ,Λqj)=Λgs(ϵ,j),p=12,q=34.
(54) In terms of p and q [28], the critical exponets read
α=2p−1q,
(55) β=1−qp,
(56) γ=2q−1p,
(57) δ=q1−q.
(58) The above scaling relations can be considered as consistency checks for the critical exponents we obtained in this section.
-
In standard black hole thermodynamics, the phase structure on the
(P,V) section has been investigated [17−19, 23, 29]. In this section, we study the phase structures on the (P,ˆV ) section in modified black hole thermodynamics. In Fig. 5, we plot theP−ˆV diagram corresponding to the Kerr-AdS black hole.
Figure 5. (color online) P-
ˆV diagram of the Kerr-AdS black hole withJ=1 . The brown solid line is the critical isotherm, and the red dot is the critical point.Following the method used in [29], after neglecting all higher order terms of J, the equation of state can be written as
P=3√π6ˆT3√ˆV−12 62/33√πˆV2/3+2πJ2(43√6π2/3ˆT3√ˆV+3)3(3√6π2/3ˆTˆV4/3+ˆV)2,
(59) where
ˆV satisfiesˆV=4πr3+3+48π(4πPr2++1)J2r+(8πPr2++3)2.
(60) Following the critical point condition
∂P∂ˆV|TC=0,∂2P∂ˆV2|TC=0 , we can obtainPc=0.003J,ˆVc=129.603J3/2,ˆTc=0.041√J.
(61) Consider the phase transition that occurs below the critical temperature
ˆTc . In Fig. 5, a van der Waals-like phase structure can be observed. The behavior of physical variables near the critical point is quantitatively described by the critical exponents. Following a similar method as in Section IV, we obtainα=α′=0,β=12,γ=1,δ=3.
(62) The above results show that the phase structure for the modified black hole thermodynamics in the
(P,ˆV) section is almost the same as the standard one [30, 31]; the only difference is that all thermal quantities have a deformation factor1√Ξ , which is shown in Eq. (7). -
In Kerr-AdS spacetime, there exist two sets of the first law of thermodynamics because of different choices of observers at infinity. In particular, we investigated the critical phenomena of black hole thermodynamics associated with rotating observers. Based on the modified first law and mass formula, we obtained the phase structures in the extended phase space for both the
(ˆΩH,J) cross-section and(P,ˆV) cross-section. In comparison with previous results [16], the phase structure within the(ˆΩH,J) plane remained analogous to the van der Waals-like phase structure. However, the difference was in the correspondence of thermodynamic quantities. According to a previous study [16], the correspondence isJ→P ,ΩH→V , but in our case, it isˆΩH→P,J→V . The aforementioned changes result from differences in observers. Compared to non-rotating observers, the energy measured by rotating observers includes a rotational kinetic energy part∼ˆΩHJ . This variation is equivalent to performing a Legendre transformation in the extended phase space with respect to the conjugate coordinates J andˆΩH ; thus, the thermodynamic quantities correspond differently. Additionally, we discussed the phase structures on the(P,ˆV) plane in modified thermodynamics. We found that the obtained results closely resemble those in standard thermodynamics. -
The authors thank Xiaokai He and Naqing Xie for their useful advice. The authors also thank the referees for their valuable comments and suggestions. These opinions have greatly enhanced the quality of this paper.




 Abstract
Abstract HTML
HTML Reference
Reference Related
Related PDF
PDF














 DownLoad:
DownLoad:






























































































































 DownLoad:
DownLoad: